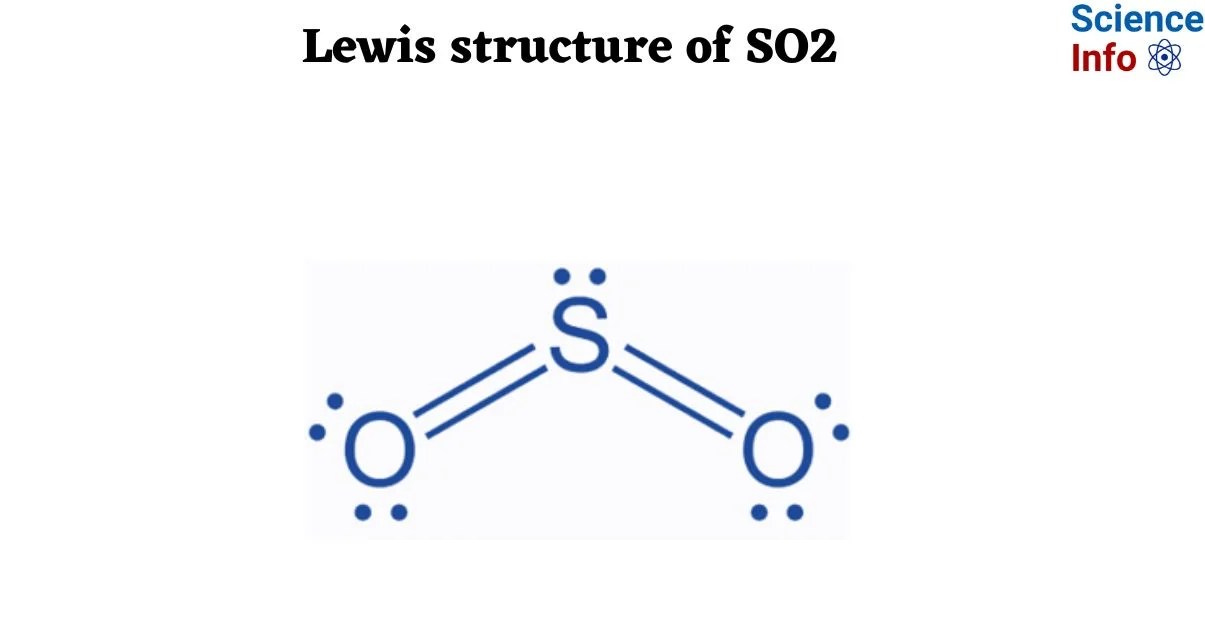
The visualization of electron distribution within a molecule, as depicted by a Lewis structure, offers fundamental insight into chemical bonding. For sulfur dioxide (SO2), this structural representation involves placing sulfur as the central atom, bonded to two oxygen atoms. The process necessitates accounting for all valence electrons, forming single and double bonds, and allocating lone pairs to achieve octets for all atoms where applicable. Crucially, SO2 exhibits resonance structures, meaning its true electron distribution is an average of multiple valid Lewis structures, contributing to its unique chemical properties and VSEPR geometry (bent molecular shape). Mastering this depiction for SO2 serves as a foundational exercise in understanding molecular architecture and electron arrangement.
Understanding Lewis structures, particularly for molecules like SO2, holds significant importance in chemistry. It provides a predictive framework for molecular geometry, polarity, and potential reactivity, which are essential for explaining a molecule's physical and chemical behavior. The ability to accurately draw and interpret these diagrams represents a critical analytical skill, fostering an innate understanding of how atoms interact to form compounds. This foundational knowledge, attributed in part to the pioneering work of Gilbert N. Lewis, is not merely an academic exercise but a practical tool that underpins advanced chemical concepts and problem-solving, cultivating an essential chemical intuition.
Proficiency in constructing and interpreting Lewis structures, exemplified by the sulfur dioxide molecule, is a pivotal step in developing a comprehensive grasp of chemical principles. This skill transcends simple diagramming; it is a gateway to predicting molecular interactions, understanding reaction mechanisms, and designing new materials. By engaging with these fundamental representations, individuals cultivate the analytical prowess necessary to navigate the complexities of molecular science, thereby empowering a deeper engagement with the subject matter and preparing for explorations into more intricate chemical phenomena.
Frequently Asked Questions Regarding Sulfur Dioxide Lewis Structures
This section addresses common inquiries and clarifies crucial aspects concerning the construction and interpretation of Lewis structures for sulfur dioxide (SO2), a fundamental exercise in chemical understanding.
Question 1: What is the established procedure for deriving the Lewis structure of sulfur dioxide (SO2)?
The process commences with calculating the total number of valence electrons for the molecule: six from sulfur and six from each of the two oxygen atoms, totaling 18 valence electrons. Sulfur is designated as the central atom due to its lower electronegativity. Single bonds are then formed between the central sulfur atom and each oxygen atom. The remaining electrons are distributed as lone pairs to satisfy the octet rule for the terminal oxygen atoms first, followed by the central sulfur atom. Often, this requires forming double bonds to achieve octets for all atoms and minimize formal charges.
Question 2: Why does SO2 exhibit resonance structures, and what is the significance of this phenomenon?
SO2 exhibits resonance because its electron distribution cannot be accurately represented by a single Lewis structure. Two equivalent resonance structures exist, involving a single bond and a double bond between the central sulfur atom and each oxygen atom, with the double bond shifting its position. This signifies that the actual bonding in SO2 is an average of these contributing structures, resulting in delocalized pi electrons and bond lengths that are intermediate between a typical single and double bond. Resonance provides a more accurate representation of electron distribution and molecular stability.
Question 3: How does the Lewis structure of SO2 determine its molecular geometry?
The Lewis structure, by illustrating the arrangement of bonding and non-bonding electron pairs around the central atom, directly informs the molecular geometry via Valence Shell Electron Pair Repulsion (VSEPR) theory. For SO2, the central sulfur atom possesses two bonding domains (to two oxygen atoms) and one lone pair, resulting in three electron domains. These domains arrange themselves in a trigonal planar electron geometry. However, the presence of the lone pair repels the bonding pairs more strongly, distorting the ideal trigonal planar arrangement and resulting in a bent (or angular) molecular geometry with an approximate bond angle of 120 degrees.
Question 4: What is the role of formal charges in evaluating the most plausible Lewis structure for SO2?
Formal charges are a conceptual tool used to assess the distribution of electrons within a molecule and identify the most stable or plausible Lewis structure among multiple possibilities. They are calculated for each atom by subtracting the number of non-bonding electrons and half the number of bonding electrons from its group number (valence electrons). For SO2, the most plausible resonance structure will typically minimize formal charges on all atoms, ideally resulting in zero or near-zero formal charges. Structures with smaller formal charges, especially with negative charges on more electronegative atoms, are generally favored.
Question 5: What are common misconceptions or errors encountered when constructing the SO2 Lewis structure?
Frequent errors include miscounting total valence electrons, incorrectly identifying the central atom, failing to apply the octet rule (or recognizing exceptions for sulfur), and neglecting to consider resonance. Another common mistake involves not minimizing formal charges, which can lead to proposing less stable or inaccurate structures. Overlooking the lone pair on the central sulfur atom, which is crucial for determining its bent geometry, also constitutes a significant error.
Question 6: Beyond simple depiction, what broader chemical insights are gained from mastering the SO2 Lewis structure?
Mastering the SO2 Lewis structure provides fundamental insights extending beyond basic representation. It forms the basis for predicting molecular polarity (SO2 is polar due to its bent shape and unequal bond dipoles), understanding its reactivity, and explaining its physical properties, such as boiling point and solubility. This foundational skill cultivates a deeper intuition for electron movement and bonding principles, which is indispensable for comprehending reaction mechanisms, intermolecular forces, and the design of novel chemical compounds. It is a critical step in developing a comprehensive chemical perspective.
The accurate derivation and interpretation of Lewis structures, exemplified by sulfur dioxide, are indispensable for developing a robust understanding of molecular properties and behavior. These fundamental models serve as critical stepping stones in chemical education and research.
Further exploration into the implications of molecular geometry and polarity will build upon the foundational concepts established through Lewis structures.
Tips for Mastering SO2 Lewis Structures
A systematic approach to Lewis structures, particularly for molecules like sulfur dioxide (SO2), is paramount for developing a robust understanding of molecular architecture and bonding. The following guidelines are designed to enhance proficiency, thereby sharpening analytical skills essential for deeper chemical comprehension.
Tip 1: Adhere to the Valence Electron Count Rigorously.The initial and most critical step involves accurately summing all valence electrons from each atom within the molecule. For SO2, sulfur contributes six, and each oxygen contributes six, totaling 18 valence electrons. Any miscalculation at this stage propagates errors throughout the entire structural derivation.
Tip 2: Identify the Central Atom Logically.The central atom is typically the least electronegative element, excluding hydrogen, or the atom that can form the most bonds. In SO2, sulfur is the less electronegative atom compared to oxygen and is thus positioned centrally, facilitating bond formation with the two oxygen atoms.
Tip 3: Prioritize Octet Fulfillment and Recognize Exceptions.Initially, distribute electrons to satisfy the octet rule for all terminal atoms, followed by the central atom. While oxygen strictly adheres to the octet rule, sulfur, being in the third period, possesses d-orbitals and can expand its octet. This flexibility is crucial for minimizing formal charges in SO2, allowing for more than eight electrons around the sulfur atom if necessary.
Tip 4: Systematically Account for Resonance Structures.For molecules like SO2, a single Lewis structure often fails to represent the true electron distribution accurately. Awareness of resonance necessitates drawing all plausible contributing structures where pi-electron density can be delocalized. In SO2, this involves alternating the position of the double bond between the central sulfur and one of the oxygen atoms, leading to two equivalent resonance forms.
Tip 5: Utilize Formal Charges for Plausibility Assessment.Formal charges serve as a diagnostic tool to evaluate the stability and plausibility of a Lewis structure. Structures with minimized formal charges (ideally zero) on all atoms, or with negative formal charges residing on more electronegative atoms, are generally considered more stable and representative of the molecule's actual electron distribution. The most accurate SO2 structures will strive for this minimization.
Tip 6: Connect Lewis Structures to Molecular Geometry (VSEPR Theory).The arrangement of bonding and non-bonding electron domains depicted in a Lewis structure directly predicts the molecule's three-dimensional geometry via Valence Shell Electron Pair Repulsion (VSEPR) theory. For SO2, the presence of two bonding pairs and one lone pair on the central sulfur atom results in a bent molecular geometry, a critical inference from its Lewis representation.
Mastery of these principles transforms the understanding of basic chemical bonding into an intuitive grasp of molecular properties and behavior. This foundational skill set is indispensable for advancing in the study of chemistry.
Further investigations into bond energies, molecular orbital theory, and spectroscopic analysis build directly upon the robust understanding developed through proficient application of Lewis structural analysis.
Conclusion
The comprehensive exploration of the sulfur dioxide (SO2) Lewis structure serves as a pivotal exercise in fundamental chemical understanding. It has been demonstrated that the systematic application of principles such as valence electron summation, central atom determination, judicious electron distribution, consideration of resonance phenomena, and formal charge evaluation is indispensable. These processes collectively yield a robust representation of molecular bonding, which, for SO2, reveals its bent molecular geometry and polar nature. The ability to accurately derive and interpret such structures forms the bedrock upon which more complex chemical concepts are built, illustrating the intrinsic connection between electron arrangement and molecular properties.
The mastery attained through rigorous engagement with such foundational concepts is transformative. The precise construction and interpretation of the SO2 Lewis structure unlock an inner chemist, fostering an acute analytical capability essential for deciphering molecular complexities. This foundational skill set provides the indispensable tools for comprehensive chemical analysis, enabling a profound connection with the fundamental principles governing molecular existence and interaction. Such expertise is not merely academic; it empowers individuals to engage deeply with chemical phenomena, predict molecular behavior, and ultimately contribute to scientific inquiry and innovation.