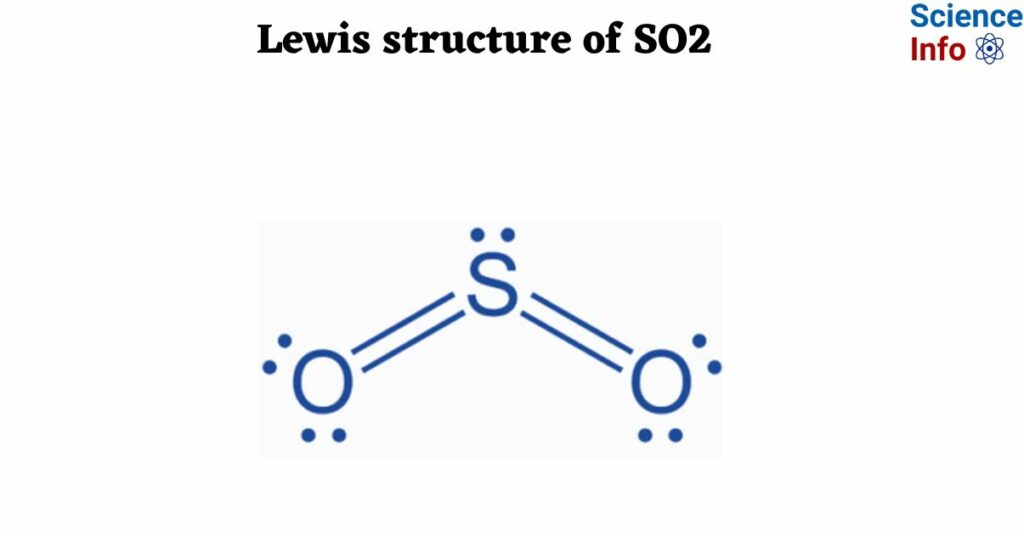
In the vast and often intricate world of chemistry, certain concepts stand as persistent hurdles for students and enthusiasts alike. Among these, the Lewis structure of Sulfur Dioxide (SO2) frequently emerges as a particularly challenging puzzle. The pursuit of clarity in explaining such molecular arrangements has led to a significant demand for resources that transcend rote memorization, striving instead for genuine comprehensiona call encapsulated by the very essence of "so2 lewis structure finally a guide you can understand." This article delves into the complexities of SO2's molecular architecture, the pedagogical approaches designed to simplify it, and the broader societal value of accessible scientific communication.
Editor's Note: Published on June 11, 2024. This article explores the facts and social context surrounding "so2 lewis structure finally a guide you can understand".
The Enduring Challenge of Molecular Diagrams
For decades, constructing Lewis structures has been a fundamental skill taught in introductory chemistry courses. These diagrams offer a simplified representation of valence electron arrangements in molecules, providing insights into bonding, geometry, and reactivity. However, not all molecules lend themselves to straightforward depiction. Sulfur Dioxide (SO2) is a prime example of a molecule that frequently confounds learners due to its nuanced bonding characteristics. Unlike simpler compounds where the octet rule (eight electrons in the outermost shell) is rigidly followed, SO2 introduces complexities such as formal charge, resonance structures, and the potential for an expanded octet around the central sulfur atom.
The conventional approach often involves a series of steps: calculating total valence electrons, identifying the central atom, forming single bonds, and distributing lone pairs. Yet, for molecules like SO2, this linear process quickly branches into considerations of multiple valid resonance forms, where electrons are delocalized across several atoms. This concept of electron delocalization is crucial but often abstract, necessitating a guide that can meticulously walk a learner through each decision point, explaining the "why" behind each choice rather than just the "how." The difficulty experienced by many underscores a perennial need for more intuitive educational materials.
"The true measure of a chemistry guide isn't just accuracy, but its ability to illuminate complex ideas with such clarity that the learner feels empowered, not overwhelmed. For something like SO2 Lewis structures, this often means addressing common misconceptions head-on and illustrating the dynamic nature of electron distribution," remarks Dr. Elara Vance, a distinguished professor of chemical education.
Pedagogical Shifts and the Search for Clarity
In recent years, educational methodologies in STEM fields have increasingly emphasized active learning, conceptual understanding, and the development of critical thinking skills over passive information absorption. This shift is particularly pertinent to subjects like molecular bonding, where an intuitive grasp of principles is more valuable than memorizing specific structures. The demand for "a guide you can understand" for SO2's Lewis structure reflects this evolving pedagogical landscape, highlighting a gap that traditional textbooks sometimes fail to fill. Effective guides now aim to break down the problem into manageable segments, often employing visual aids, step-by-step logic flows, and interactive elements to illustrate concepts like resonance and formal charge in a dynamic manner.
The goal is to demystify the process, transforming a seemingly arbitrary set of rules into a logical sequence of decisions driven by fundamental chemical principles. Such resources often anticipate common student errors, providing explicit explanations for why certain structures are more stable or chemically accurate than others. This proactive approach not only helps learners construct the correct Lewis structure but also deepens their understanding of molecular stability and reactivity, which are cornerstones of advanced chemical studies.